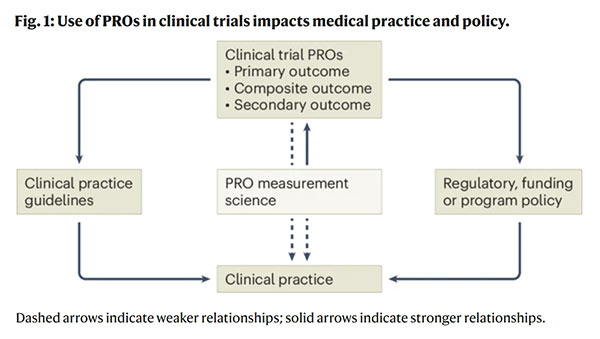
This Nature Medicine Comment discusses how including PROs as endpoints in clinical trials can influence trial outcomes, regulatory decisions, and clinical practice.
Citation Information
Crossnohere NL, Schuster ALR, Bruckel J, Chen RC, Cizik AM, Cruz Rivera S, Muth C, Nelsen L, Kyte D, Wu AW, Thorner E, Snyder C, Brundage M. Patient-reported outcome measures add value as clinical trial endpoints. Nat Med. 2025 Aug 25. doi: 10.1038/s41591-025-03906-1. Epub ahead of print. PMID: 40855193.