PROTEUS — TRIALS

Resources And Tools To Obtain The Patient Perspective
To conduct high-quality clinical trials and provide truly patient-centered care, it is necessary to understand patients’ experience. That is why it is valuable to obtain patients’ perspectives about their symptoms, functioning, and well-being.
Patient-reported outcomes (PROs) have become a central component in clinical trials. Research has shown that patients value PRO data from clinical trials to understand the ways that treatments affect how they feel, function, and live their lives, and to help make treatment decisions. Clinical trial PRO results can inform clinical practice, regulatory decisions, and policy (see examples in this JNCI Commentary).
To ensure patients, clinicians, and other decision makers can get high-quality PRO data from clinical trials, research studies must use a SMART approach:
What’s In The PROTEUS-Trials Section
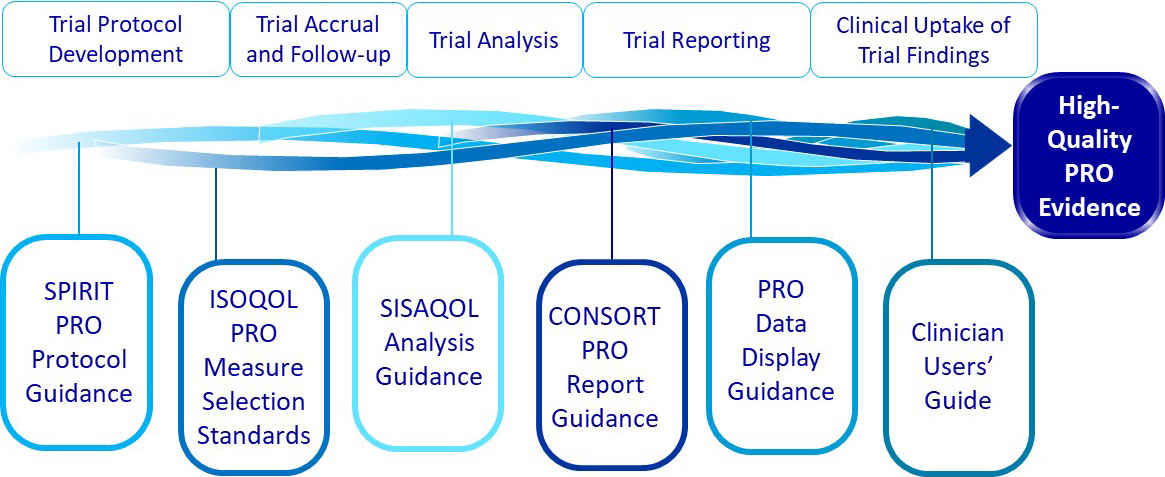
PROTEUS-Trials provides a curated collection of core methodologic tools that inform the use of patient-reported outcome data in clinical trials.
Each page listed below represents an important step in the PRO clinical trial process. Each page also provides relevant resources and useful tools to help navigate the use of PROs for that step in the process.
A Short Communication published in Clinical Trials summarizes all of the core methodologic tools.
In addition to the PROTEUS tools, guidance documents from the U.S. Food & Drug Administration (here) and European Medicines Agency (here and here) provide their perspectives on the use of PROMs for regulatory submissions.
PROTEUS Trials Roadmap

The PROTEUS-Trials Handbook
As a companion to the individual pages described above, the PROTEUS Handbook provides a summary of the resources and discusses why the information is needed, the objective it serves, the recommendations it contains, and other relevant context.
On the pages dedicated to each of the Trials research phases, a short introductory excerpt of the Handbook will be included among the Core Resources provided, as will the full relevant chapter of the Handbook.
You can also review the Handbook in its entirety. Find the US (letter) version here and the UK (A4) version here.
